CDISC
Clarifying a brand’s value for audiences across the healthcare spectrum
CDISC stood in a category of its own. For 20+ years, the pioneer organization was the only one focused on developing and advancing clinical research data standards across geographies, disciplines, and research studies. Recently, both the U.S. FDA and Japan PMDA made implementing CDISC’s standards a requirement, elevating CDISC’s stature across the healthcare spectrum. With this gained momentum, it became critically important to convey the value and importance of CDISC’s work to its wide array of audiences—from pharmaceutical companies to academics, regulators, patient foundations, and tech vendors.
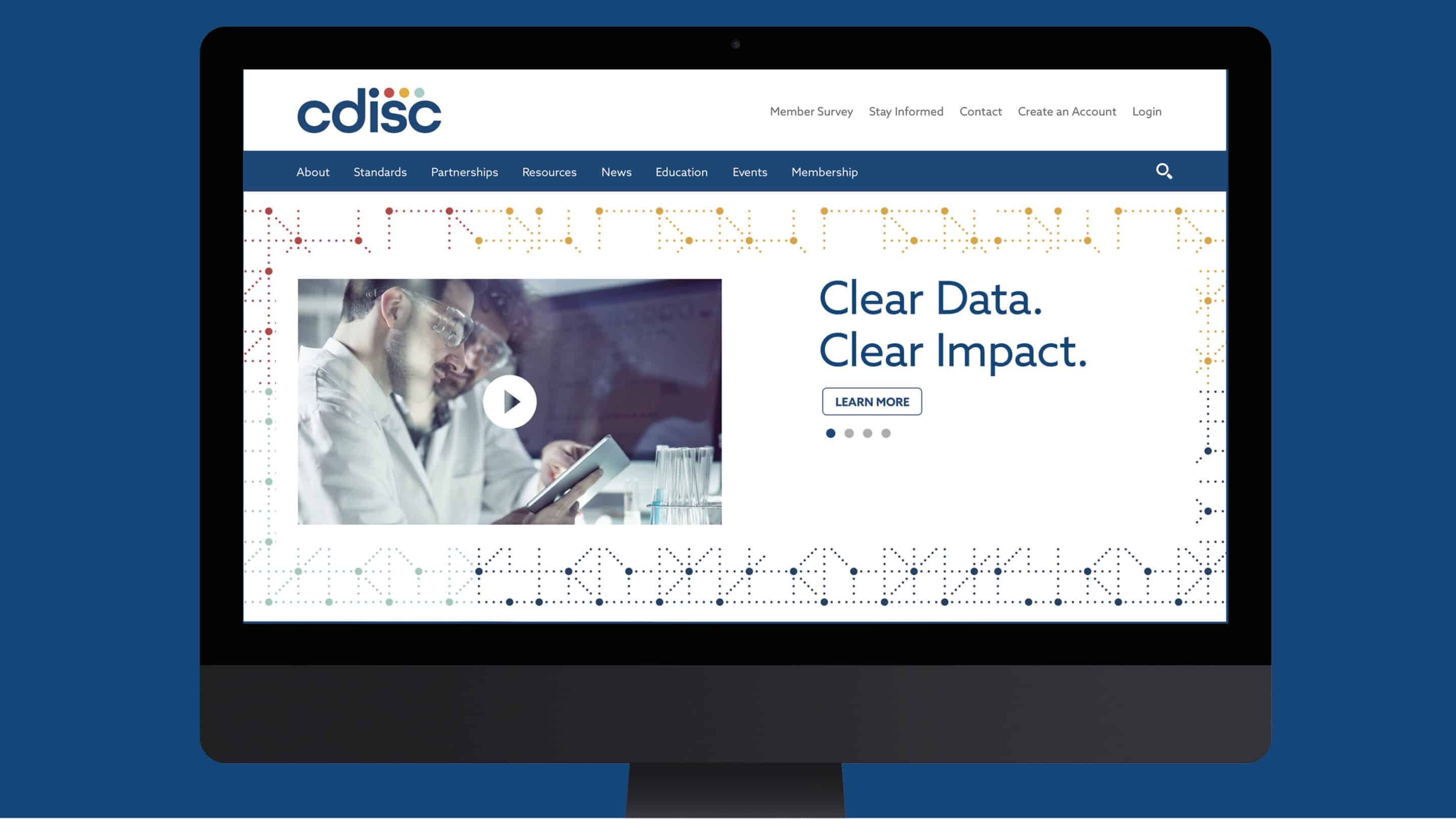
Research revealed that many of its audiences considered CDISC’s standards a burden when it came to their scientific approach. To help shift perceptions of CDISC from a required step to a desired and invaluable partner, the new brand focused on the fundamental role the organization played: the critical facilitator of clarity in clinical research data. Crystalized in a new tagline—“Clear Data. Clear Impact.”—CDISC’s value and higher purpose were brought to the forefront.
CDISC’s success is reliant on its ability to convene its community of volunteers and organizations across the globe to co-create the data standards. This cornerstone idea of collaboration was brought to life through the brand’s visual and verbal system. Throughout messaging, it worked to empower audiences, informing them that they not only have the opportunity to shape the development of the standards themselves but they also have the chance to work with peers from around the world to uncover new breakthroughs that would have otherwise remained hidden. The brand’s proprietary and dynamic pattern visualizes the interconnectedness and expansiveness of collective ideas.
A series of videos helped illustrate CDISC’s passion, mission, and sense of purpose. Through personal stories and scientific examples, CDISC employees and volunteers shared the impact they were able to make because of their involvement with the organization. The films also reinforce how CDISC brings together a global community, featuring a diverse set of individuals spanning professional competencies and languages.